What is Activated Carbon
What is Activated Carbon?
Activated Carbon is a porous material that removes organic compounds from liquids and gases by a process known as “adsorption.” In adsorption, organic molecules contained in a liquid or gas are attracted and bound to the surface of the pores of the activated carbon as the liquid or gas is passed through.
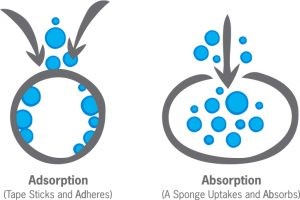
There’s a distinct difference between ADsorption and ABsorption:
The primary raw material used in the production of our activated carbons is bituminous coal that is crushed, sized and processed in low-temperature bakers followed by high-temperature activation furnaces.
The structure of our activated carbon is one of the reasons it’s considered the best in the market. It’s about what’s on the inside. Let’s take a closer look:

The structure of activated carbon is composed of graphitic plates. These plates are connected by carbon-carbon bonds, which create a porous structure and give an extensive internal surface area, which is where adsorption occurs. Adsorption is one type of Van der Waal’s force, which works on the molecular level to attract molecules to each other. With activated carbon, the surface of the carbon is attracted to the compound being adsorbed.
Intermolecular attractions in the smallest pore of the carbon particle result in adsorption forces that cause precipitation of adsorbates from solutions, and condensation of adsorbate gases.
Adsorbates actually undergo a type of phase change because they are so much lower in energy once they are adsorbed.
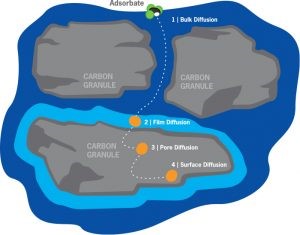
Spent activated carbon doesn’t swell like an ion exchange resin or sponge upon use. All of the work takes place inside the pores – deep inside the granules – where the water molecule at the surface is displaced by an adsorbate molecule.
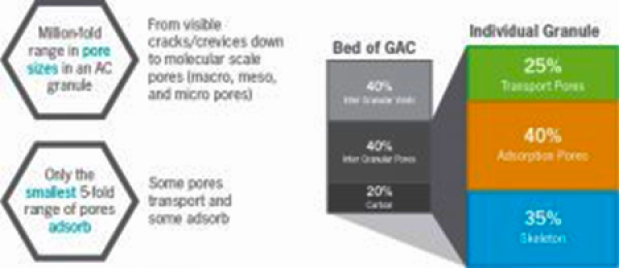
Activated carbon has a transport system with a variety of pore sizes:
From very large transport pores to smaller transport pores With ability to handle high to low adsorbate movement rates
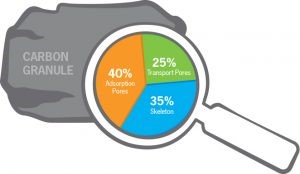
Magnifications of an activated carbon particle:
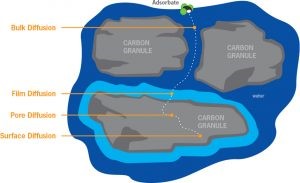
This final image shows the molecular structure of activated carbon that is saturated with adsorbate. The adsorbate needs to be close to a carbon plate to adsorb. Once the adsorbate is more than a few molecular diameters away from the carbon surface, adsorption does not occur.